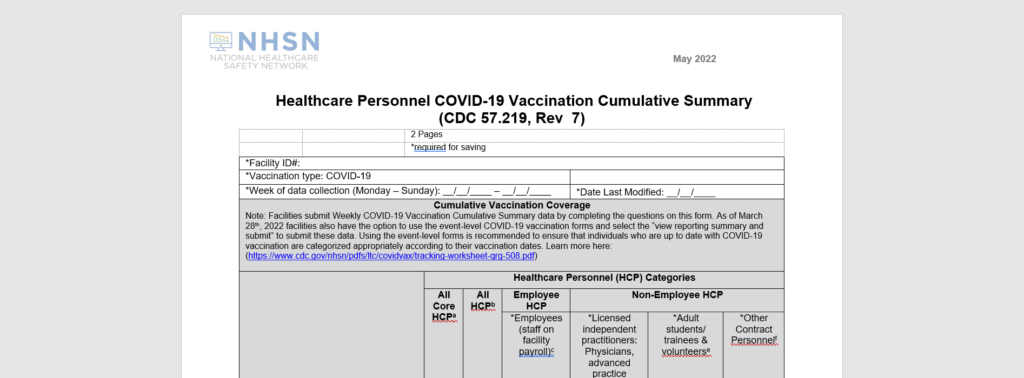
What are the main changes?
Simplifications
- COVID-19 vaccination data will no longer be reported by vaccine manufacturer for questions on primary vaccine series and additional/booster doses.
- Questions on vaccine supply will be removed.
- Completing a monthly reporting plan for the COVID-19 Vaccination Module in the Healthcare Personnel Safety Component will no longer be required. Instead, upon saving or uploading data, users will agree to the following:
- The data reported are consistent with definitions outlined in NHSN surveillance protocols (including tables of instructions and frequently asked questions).
- The data will be sent to the Centers for Medicare and Medicaid Services (CMS) to fulfill CMS quality reporting requirements (when applicable).
Additions
Adding a question for facilities to report the cumulative number of individuals who are up to date with COVID-19 vaccination.
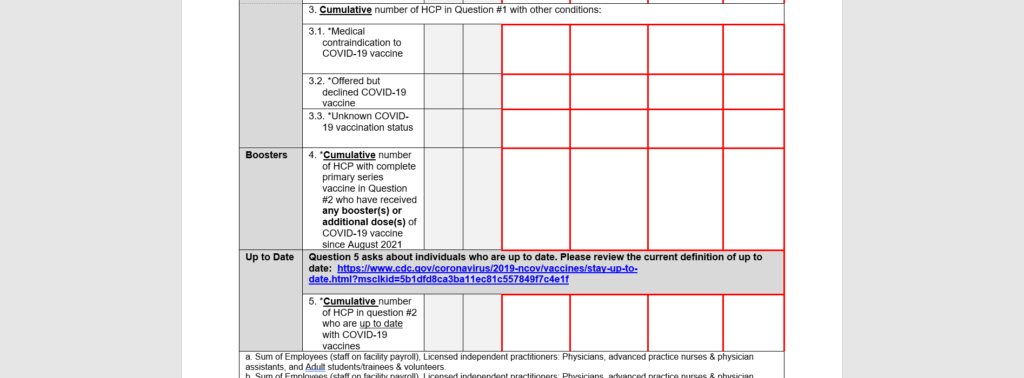
How should I prepare?
Facilities should develop or update data tracking mechanisms to collect weekly COVID-19 vaccination data on healthcare personnel for the additional questions.
How can I learn more?
CDC will conduct a training webinar to review these changes for the Healthcare Personnel Safety Component on May 25, 2022, 2:00 PM Eastern Time.
After registering, you will receive a confirmation e-mail containing information about joining the webinar.
If you have questions, please send an e-mail to NHSN@cdc.gov with ‘COVID-19 Vaccination Data Reporting’ in the subject line.


